A ternary phase diagram has three components. The three components are usually compositions of elements, but may include temperature or pressure also. This type of diagram is three-dimensional but is illustrated in two-dimensions for ease of drawing and reading. Instead of being a rectangular plot, it is a triangle. Ternary phase diagrams exist for many metallic alloys, but are also widely used in ceramics. Stainless steel (Fe-Ni-Cr) is a perfect example of a metal alloy that is represented by a ternary phase diagram. Stainless steel is a very common metal alloy. Almost everyone knows of an everyday object that is made with stainles steel. In the figure below stainless steel has been used to construct a portable hoist. You can find out more about other products made from stainless steel via the link provided in the Fig. 1 caption.

Ternary phase diagrams are needed so that three components can be compared at once. For example, stainless steel has iron, nickel, and chromium compositions. To view all three compositions at the same time, a triangular plot is set up with an element at each of the vertexes with the temperature and pressure stated. In ceramic systems, sometimes compounds are located at the vertexes instead of elements. The derivation of the ternary plot is too complicated to go into, but the analytical derivation of a binary system is available along with the experimental method of determining the phase diagram
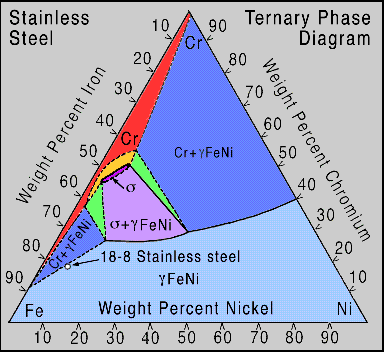
Reading the compositions of iron, chromium and nickel at any point on the stainless steel ternary phase diagram in Fig. 2 is simple. Instead of drawing one tie-line, as in a binary phase diagram , three lines are drawn, each parallel to a side of the triangle and going through the point in question. Extend the lines so they pass through an axes. To find the iron composition, the line drawn parallel to the axis opposite the Fe vertex is the one needed. The percent iron is then read off the axis.
For example, to determine the compositions of the 18-8 circle point near the lower left corner of Fig. 2, draw these lines: 1. draw the first line to be parallel with the axis opposite the Fe vertex, we find that the composition of iron is 74% , 2. next draw a line parallel with axis opposite the Ni vertex and read the composition of nickel to be 8% , 3. and finally draw a line parallel to the axis opposite the Cr vertex and we see that there is 18% of chromium at that point. The point described is then called 18-8 stainless steel, naming only the percentages of the chromium and the nickel; the iron content being dependent on the other two. This 'recipe' for stainless steel is the most common.
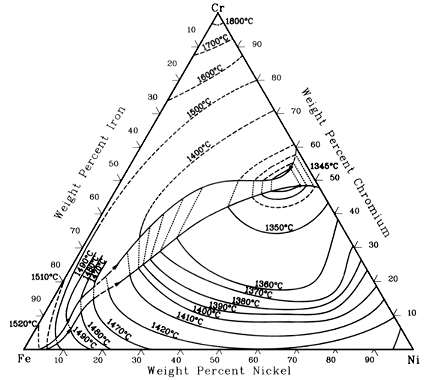
Figure 3 represents the entire ternary phase diagram solidus projections of stainless steel over a range of temperatures under constant pressure. This picture illustrates how temperature affects solubility.
Before now, the pressure was kept constant (1 atm), which is acceptable for most applications but in extreme pressure environments, a scientist must consider the pressure dependence of a system. The variance of pressure brings about a whole new dimension -- the fourth dimension. The pressure is usually not taken into consideration unless a gas phase is liable to be present.
For information on the materials class that this subject is studied in, visit the MSE 3424 syllabus, Phase Equilibria and Crystal Chemistry.
http://www.eng.vt.edu/eng/materials/classes/MSE2094_NoteBook/
96ClassProj/experimental/ternary2.html