Development of Iron/Carbon Compositions

Introduction
Before any determination of steel phases or microstructure can be attempted
there must be a way to obtain samples of different iron/carbon compositions.
Industry generally uses two methods: diffusion and
individual compostition production. If the compositions are not correct then any data
gathered will be faulty. Constructing a full phase diagram by these methods can take one year for a two component system, three years for three components, and up to ten years for four components, so workers must show great care to get exact composition for a small margin for error.
In either method starting material purity is very important. For example steel production requires graphite carbon and iron. There must be no impurities in either of the constituents. Even the smallest impurity can cause the sample to be much different than that of the phase-equilibrum relations that were intended to be studied. Care must also be taken to assure that none of the instruments used to mix the different compositions will contaminate the samples, so any materials that come into contact with
the sample should not react with the sample. After the preliminary handling of the ingredients, it is time to create the different compositions.
Diffusion
Difffusion is the simplest method to create a wide spectrum of compositions at one time. A diffusion block system is set up by putting a block of 1.2 wt % carbon and another block of 99.75 wt % iron (0.25 wt% Carbon) into contact (shown in Fig.1). At a given temperature, carbon diffuses into the iron, generating a carbon gradient across the system (shown in Fig. 2). The location of different compositions can be determined through mathematical modeling of the experiment. Once a general position for the desired composition is determined, that cross-section of the block is removed and tested through x-ray flourescence to determine the relative
percents of the two elements present. This method of determining the composition is preferable since it is non-destructive. Other methods are wet chemical analysis which is the use of chemistry to find the composition and other types of spectroscopy such as the mass spectrometer. Once the composition of the sample is determined then it can be examined at different temperatures
to find the phases present. Since this method produces a limited amount of sample, it lends its self very well to determination of these phases by x-ray diffraction. If you wish to learn more about how diffusion works and how the composition gradient can be modeled, you can visit last year's (1995) diffusion project.

Figure 1. Diffusion block setup before diffusion takes place
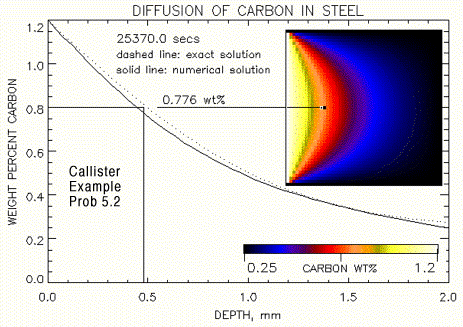
Figure 2. Composition gradient for Iron/Carbon diffusion
Specific Compositions
More detail on a certain composition requires a large amount of that composition. The specific percents of the carbon and iron can be measured out and then melted to form that composition. For example if you needed a 50 wt% carbon / 50 wt% iron compositon, you would mix even parts by weight in a manner minimizing impuities such as oxygen and nitrogen, possible by carrying out the melting in a vacuum furnace and making sure the crucible or other melting vessel is free of contaminants. This method can be used when large volumes of a certain composition are needed to do experimentation such as dilatometry or differential thermal analysis.
Conclusion
No matter which method you choose, the purity of the original components and the
threat of contamination must be a very high priority. If these two potential problems can be kept
under control, the creation of the different compositions for analysis becomes a very straight forward operation. Once these compositions are manufactured then it is time to determine which phases are present at what temperatures for what compositions. With this done we are well on our way to constructing the iron/carbon phase diagram.
Jeffrey M. Haeberle 4/28/96
http://www.eng.vt.edu/eng/materials/classes/MSE2094_NoteBook/
96ClassProj/experimental/steel.html
Project Hompage | Experimental Page



