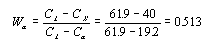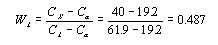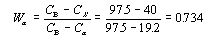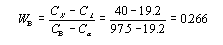This step requires the use of the lever rule since the alloy consists of two phases.
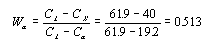
Thus the alpha phase makes up 51.3% of the alloy. There are only two phases so the sum of their mass
fractions must equal 1. Therefore the liquid phase makes up .487, or 48.7%, of the alloy. This number
could also have been calculated using the lever rule.
phase diagram
Part 2:
Now, the temperature is lowered slightly to right below the eutectic line at 182.9%.
- a) what is the composition of each phase?
- b) what is the relative amount of each phase present, in mass fraction?
a)
Drop the lines from the intersections as before to discover that the composition of the alpha phase
is 19.2 wt% Sn and the composition of the beta phase is 97.5% wt% Sn.
b)
The lever rule is used again.
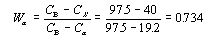
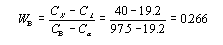
Thus the mass fraction of the alpha phase is .734, or 73.4%, and that of the beta phase is .266, of 26.6%.
Clickable Image
Part 3:
The alpha phase can be divided into two identifiable parts. The alpha phase described in Part 1 of this
problem does not change in any way as the temperature is lowered. It is termed the proeutectoid alpha.
However, as the temperature decreases, the liquid phase at the eutectic point solidifies into a standard
eutectic structure consisting of alternating layers of the alpha and beta phases. The alpha phase found
in this structure is termed the eutectic alpha. The only difference between the two types of the alpha
phase is their place in the structure.
Using these definitions, what is the mass fraction of these phases at the conditions of Part 2?
- a) proeutectic alpha
- b) eutectic alpha
- c) beta
The mass fraction of alpha compared to the mass fraction of liquid was determined in Part 1.
This is the proeutectic alpha and is 0.513 of the whole. The total mass fraction of alpha was determined
in Part 2 as being 0.734. Since the total amount of alloy did not change, the amount of eutectic alpha must
be 0.734 - 0.513 = 0.221 of the whole. The mass fraction of beta was also determined in Part 2 to be .266.
- a) 0.513
- b) 0.221
- c) 0.266
An application of the lead-tin alloy is solder.
Beth Oborn 4/26/96
http://www.eng.vt.edu/eng/materials/classes/MSE2094_NoteBook/
96ClassProj/examples/bethcon.html
Project Hompage | Examples Page