The Copper-Nickel Application
The Copper-Nickel alloy has been widely used since very early times, when a durable material for coin production was needed ("Nickel"). The Copper-Nickel system has very unique properties, primarily that each component is completely soluble in the other. In other words, at any composition and temperature, the phases occurring are compositionally homogeneous and composed of both Cu and Ni. Such a system is termed isomorphous (Callister 242). Below, is the phase diagram for the Cu-Ni system.
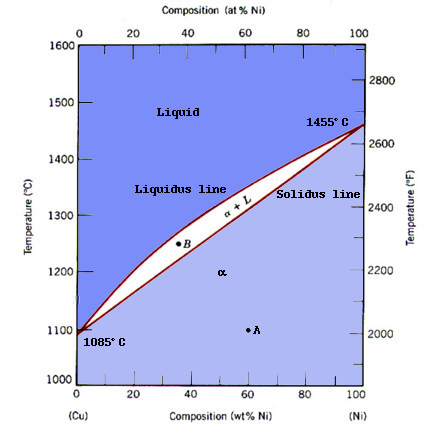
Notice that the phase diagram contains three regions. In the upper region, the material consists of a liquid solution of Cu and Ni. The middle region contains both liquid and solid phases, whose composition may be calculated using the lever rule. To see an example, click here. The lower region is the one we are most interested in when we talk about applications. In this region, the Cu-Ni is a substitutional solid solution, where copper and nickel atoms interchangeably form the crystal lattice. A substitutional solid solution occurs in Cu-Ni because: Cu and Ni both crystallize in the face-centered cubic structure, have similar atomic radii, electronegativities, and valences (Callister 242).
Individually, Copper and Nickel have different characteristics. Copper is a soft, ductile, highly conductive, nonreactive, material which melts at a temperature of 1085Co whereas, Nickel is a relatively hard, corrosion resistant metal that melts at 1455Co. Using these properties, Materials Scientists have alloyed Copper-Nickel at different concentrations to obtain the best characteristics for practical use.
The Copper-Nickel alloy is found in numerous applications of technology. Cu-Ni tends to be very resistant to corrosion, and as a result, Cu-Ni piping is used in many sea-water applications. In distillation plants, Cu-Ni containers resist the corrosive effects of boiling sea-water ("Alloy"). Many of the wires and electric contacts on sea faring vessels, are made of Cu-Ni, where the addition of Nickel only partially reduces the conductivity of Copper. One example of Cu-Ni in the textile industry is large vessels made of acid resistant Cu-Ni that contain many acidic dyes and solutions ("Dyeing"). Finally, the addition of Ni raises the melting point of Cu alone. This alloy can replace Cu wire in environments where the temperature can be very high. The Copper-Nickel alloy has proven to be very useful for its ability to withstand corrosive environments. Because if its good characteristics, and its relatively inexpensive processing, Copper-Nickel will continue to hold an important place in many scientific and practical applications.
Garrett Storaska 4/28/96
http://www.eng.vt.edu/eng/materials/classes/MSE2094_NoteBook/
96ClassProj/examples/cuni.html
Project Homepage | Examples/Applications group

